OVERVIEW
Timeline: September - May 2018 (8 months)
Focus: Engineering | Mechanical design | Medical devices | Manufacturing
Collaborators: Quinn Coyle | Eli Gottlieb | Caroline Landon | Emily Pugh | Daniel Shin
Faculty and technical advisors: Dr. Graham E. Wabiszewski | Dr. Paulo E. Arratia | Dr. Collin T. Stabler
I applied my engineering knowledge in this year-long capstone mechanical engineering project, in collaboration with five teammates. We designed a biomimetic chest cavity that houses lungs between harvest and transplant, aiming to minimize injury of donor lungs by simulating the movement of lungs inside the human body. Lungevity’s key system components include an actuated chest cavity and diaphragm that drive the volume change for negative pressure breathing and a custom-molded silicone outer membrane that surrounds the chest cavity and provides a flexible seal. The system was designed as a proof-of-concept for researchers to be able to further investigate the improvement of lung viability in a negative pressure, confined system versus a system with applied positive pressure. Dr. Collin Stabler sponsored the project and provided inspiration and anatomical guidance during the ideation phase. My role on the team focused on the diaphragm and seal design and fabrication.
PROCESS
System employs near-physiological confinement and negative-pressure breathing
The greatest challenge in this project was designing a closed system that is simultaneously flexible, allowing for volumetric change inside. Additionally, through research we justified that to mimic “breathing”, in a way that doesn’t inflict damage to the lungs, more is necessary than a simple volumetric change resulting in a change in pressure; the way in which the volume is changes is significant in lung preservation. Therefore, we set a goal to create regional volume changes as close as possible to a human chest cavity by designing for biomimetic shape and movement. Thus, the human body served as the basis for the primary defining characteristics of the system.
DESIGN
At the center of the system are the donor lungs that we are trying to preserve. The lungs are confined by two biomimetic structures: the chest cavity and the diaphragm. These two components change the volume in the chest cavity through movement driven by linear actuators. The movement leads to a change in volume which results in a change in pressure.
System sealing
A three-flange system was designed for the purpose of creating an airtight system that allows for insertion and removal of the lungs. The flanges are designed to clamp the silicone of the outer membrane and of the diaphragm. The outer membrane seals between the top and middle flanges and the diaphragm seals between the middle and bottom flanges.
A flexible silicone outer membrane allows for volumetric change of the system while simultaneously sealing the system. The membrane is specifically designed to create a way for all of the external interfaces to be sealed. This includes: the trachea interface, the vacuum port interface, the two interfaces between the linear actuators and the chest cavity, the “spine” back interface, and the flange interface. At each interface, the silicone is clamped to create an airtight seal.
Diaphragm design
The three key components for the diaphragm design include: actuation mechanism, shape, and seal. We conceptualized a variety of ways to achieve each of these three components and combinations of them. Ultimately, we determined that a linear actuator would be the best actuation mechanism and the shape would be biomimetic, partially rigid and partially flexible, extending out to a membrane which can clamp between flanges in order to seal the bottom of the chest cavity.
FABRICATION
Diaphragm
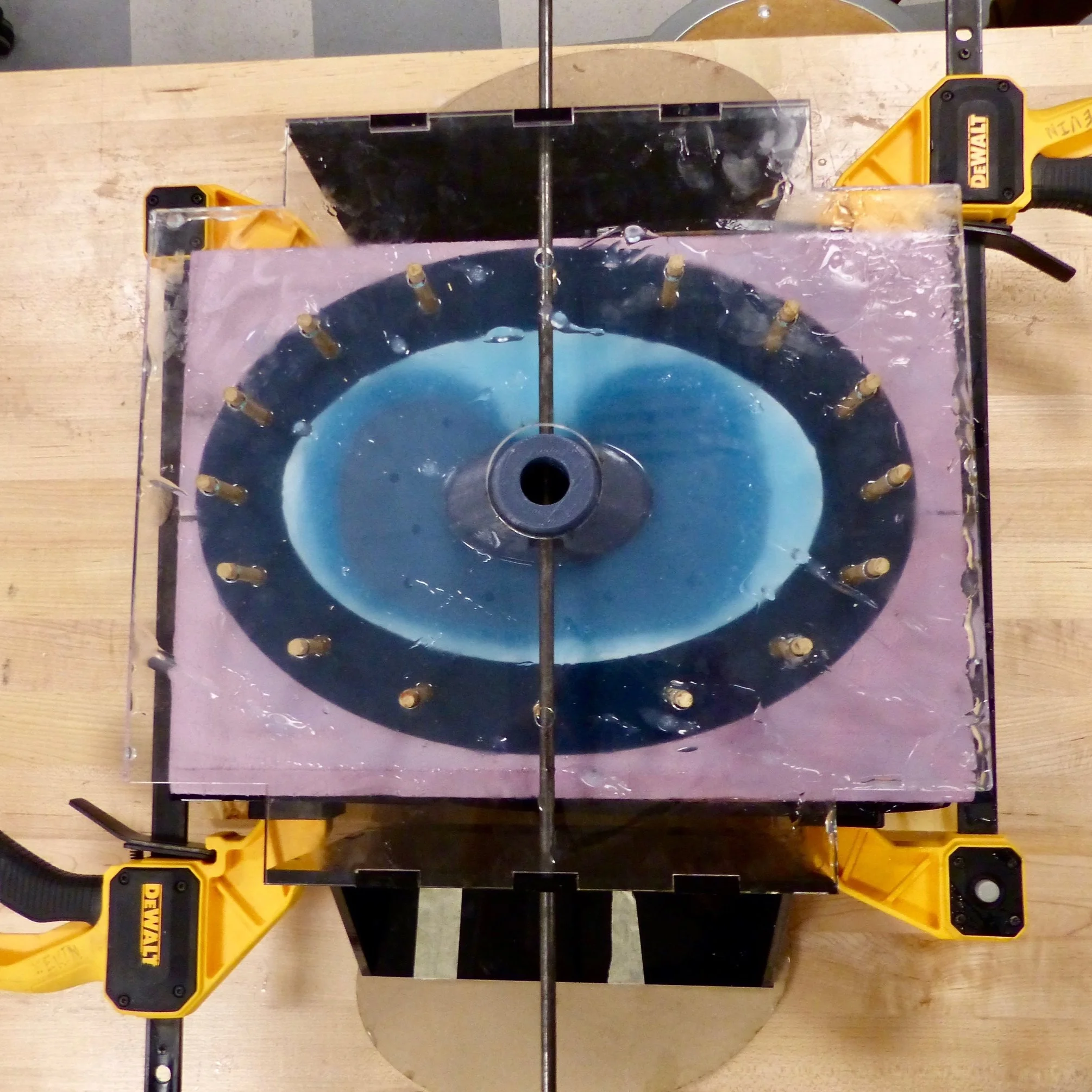
Due to the shape specificity, it was necessary for us to fabricate a custom mold. The mold shape was CNC milled out of foam and then cut into two pieces. The mold was designed such that a scaled-down 3D printed piece is suspended on an axle from a particular height such that silicone can surround the substrate uniformly with a desired thickness.
Outer membrane
A custom mold was also designed for the outer membrane, which was fabricated in pieces, due to geometry and later combined.
VALIDATION
Testing
We defined three mechanical metrics to validate the system: breathing rate, pressure, and strain. While these metrics may not directly indicate lung damage, matching them with the system is critical to ensure that the system environment closely matches that of a human body. We tested our system using pig lungs, which are very similar to human lungs and are commonly used in medical research.
Validating metrics within the system required creativity due to complex environmental conditions and available resources. A vacuum pump was attached to the system to initialize the pressure inside of the chest cavity. Two manometers were used to measure pressure at the upper and lower portions of the chest cavity. Manometer ports integrated into the outer membrane design so that the manometers could it would be possible to access the chest cavity without compromising the seal of the system. The team designed a specialized system to determine whether unsafe strain was experienced during testing.